Esperovax and Life Magnetics are working to address the big issue with RNA therapeutics
On August 1st, 2022, we issued a press release on our collaboration with Esperovax. A copy can be found here.
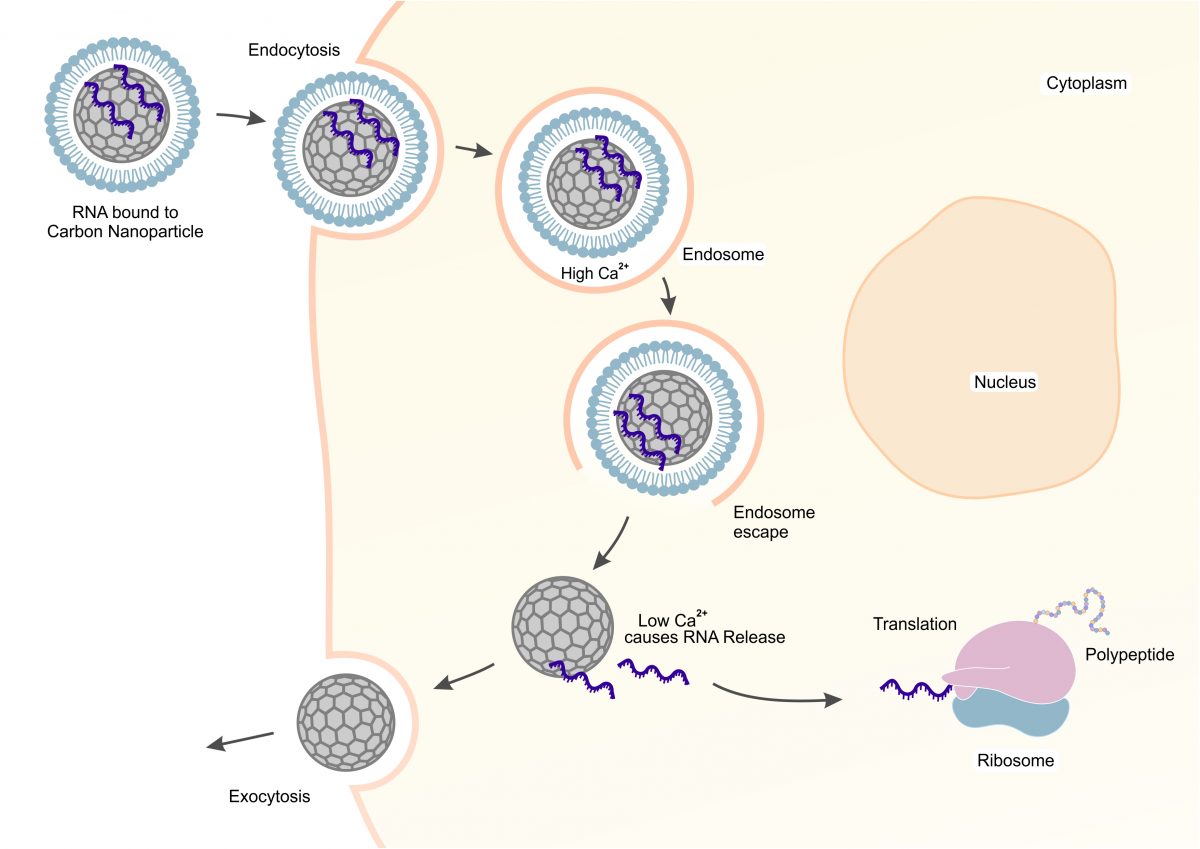
In this post, we wanted to go a bit more in-depth into what comes next in the development process. The headline image depicts how this technology delivers RNA therapeutics. The key steps in this process have been verified. We were able to show that we can load and stabilize RNA on the carbon nanoparticles using Ca2+.
When the Ca2+ levels fall in the cell, the RNA releases and translates into proteins. We’ve also shown that there is no toxicity when animals are fed high doses of the carbon. Mice fed high doses of the carbon showed no ill effects, the carbon passed their digestive system. The big challenge remaining is designing the lipid layer. This is a critical step because it enables endosomal escape. The good news is our beads are complimentary to many existing technologies that do this.
Successful Milestones
The key success from this collaboration was demonstrating that RNA could be delivered to cells and express proteins. RNA binds to the carbon when Ca2+ is present. Calcium is naturally present in the body and required for healthy cell function. Outside of the cell, Ca2+ concentrations are higher. Inside the cell, the Ca2+ concentration drops. This catalyzes the release of the RNA from the carbon surface.
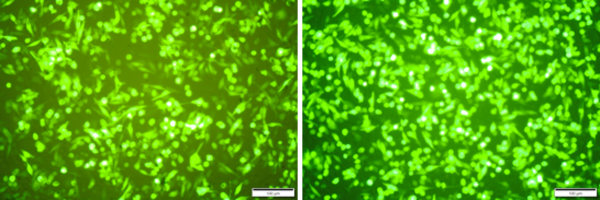
Thanks to the wonderful scientists at Esperovax, we were able to prove this key feature works as intended for delivering RNA therapeutics to cells. The images to the left show green fluorescent protein made in cells delivered using our technology (left) and the existing gold standard delivery method (right). Results are similar and this is good. Our technology also stabilizes RNA and delivering room-temperature stored RNA was the goal.
Targeting Colorectal Cancer with an RNA Therapeutics
The key remaining challenge for the beads to be used as an RNA therapeutic is developing the lipid layer which helps the beads enter cells and escape the endosome. While the RNA may be stable on the nanoparticles, the lipid layer which aids in getting the particles into the cell and out of the endosome were not stable. The lipids would fall off the beads which was an anticipated result because the carbon stabilizes RNA, not lipids. There’s an obvious fix though, conjugate the lipids to the beads directly. To successfully use this product in RNA therapeutics we need to develop the lipid coating. The good news is we don’t need to reinvent the wheel here. In fact, our end goal should be to show this technology is broadly applicable and works with a variety of technologies.
By focusing on Colorectal Cancer first, we can bypass many of the other issues. For example, is the carbon bioabsorbable? We don’t know. However, we do know the beads are expelled as waste if taken orally. That means for this application it doesn’t matter. We won’t need to answer this question for this application.
RNA therapeutics technology Pipeline
The current goal is Phase I trials in 24 months. In the first three months, we proved the project is feasible and the key science is sound. Esperovax is developing partnerships to take the technology forward. They are working with a CRO to do high throughput screening. If our technology is also amendable to high-throughput for RNA therapeutics screening, then this is also valuable to shorten drug development time.
Interested in trying this yourself?
The protocol developed is extremely simple. If you’d like to test this yourself, a protocol has been posted in the protocols section here. After binding the RNA to the beads, mixing with a lipid such as lipofectamine allows for endosomal escape and RNA delivery to cells.

Kevin graduated from the University of Michigan where he received a Doctor of Philosophy in Materials Chemistry with a focus on Semiconductor Surface Chemistry. In 2015, Kevin started Life Magnetics, Inc. with Saravana Murthy. Kevin developed the manufacturing method used to create Life Magnetics’ products and currently oversees manufacturing and business development. Kevin also has five peer-reviewed publications, and seven issued patents.